How long does it take for different countries/regions to approve new drugs? The United States is the fastest and Europe the slowest among selected major pharmaceutical markets, according to a EFPIA report.
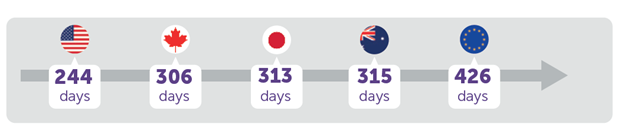
Furthermore, while the majority (7 in 10) of therapies in the US rely on accelerated approval, in Europe fewer than 1 in 10 are approved through these pathways.
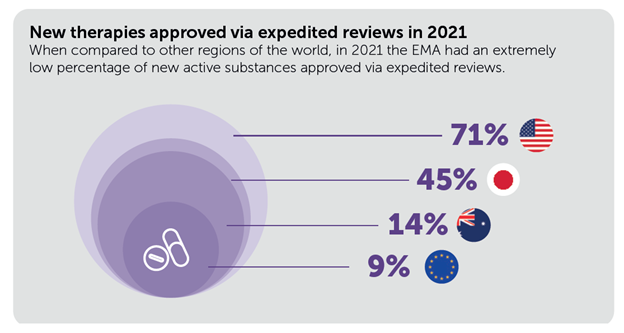
You can read the full report and EFPIA’s response to the EU pharmaceuticals package here.








